As discussed previously, water molecules are polar in nature and forms a strong network attracting each other which can be seen in the diagram below:
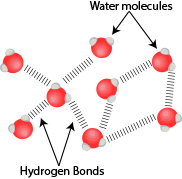
Polar water molecules attracted to each other via hydrogen bonding
Before jumping into explaing the moving pepper, we need to know a bit more about another important player in this experiment – detergent. A molecule of detergent consists of two parts – the water-loving (hydrophilic) head and the water-hating (hydrophobic) tail. This means that the head would be attracted to water molecules while the tail would try to avoid them as much as possible.
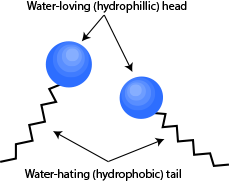
Illustration of detergent molecules
Now, try imaging what will happen when you introduce detergent molecules to this network of water molecules.
The hydrophilic head of the detergent molecules will be attracted to the water molecule and at the same time, the hydrophobic tail will be as far away as possible from the water molecules.
The detergent molecules would form an all-round barrier, with the hydrophilic head facing outwards towards the water molecules but the hydrophobic tail will be facing inwards.
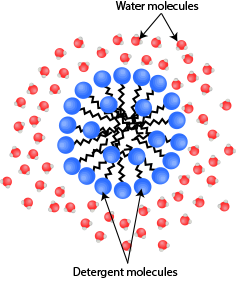
Detergent molecules forming a “ball-like shape” so that the hydrophilic head would maximise contact with water while the hydrophobic tails will minimise contact with water.
The strong network of water molecules is now broken at the point where the detergent molecules is introduced (image the network of water molecules are like the rubber surface of a blown balloon and introducing detergent is like poking the surface with a sharp needle!)

At the point where the detergent is introduced, the hydrogen bonds between the water molecules will weaken. However, the other sides that are “detergent-free” still maintain the strong hydrogen bonding and thus pull the water molecules outwards to the edge of the plate.
This motion will move the pepper particles floating on the surface along and there, you have the “balloon skin ripping motion”!

